ARTÍCULOS
AES depth profile and photoconductive studies of AgInS2 thin films prepared by co-evaporation
Estudios de perfiles de profundidad AES y fotoconductividad de películas delgadas de AgInS2 preparadas por co-evaporación
C. A. Arredondo*; C. Calderón**; P. Bartolo-Pérez***; G. Gordillo****; E. Romero*****
* Doctor en Ciencias Física, Universidad Nacional de Colombia- Bogotá. Docente del programa de Ingeniería en Energía, Universidad de Medellín. Correo electrónico caarredondo@udem.edu.co
** Autora para correspondencia. Doctora en Ciencias Física, Universidad Nacional de Colombia- Bogotá. Profesora asociada del Departamento de Física, Universidad Nacional de Colombia, Bogotá. Correo electrónico clcalderont@unal.edu.co
*** Doctor en Ciencias, Física de Materiales, Centro de Investigación Científica y de Educación Superior de Ensenada, México. Investigador del Departamento de Física Aplicada, CINVESTAV- IPN, Mérida, Yucatán, México. Correo electrónico pascual@mda.cinvestav.mx
**** Dr. Rer. Nat. Universidad de Stuttgart, Alemania. Profesor Titular del Departamento de Física, Universidad Nacional de Colombia, Bogotá. Director del Grupo de Energía Solar y Materiales Semiconductores. Correo electrónico ggordillog@unal.edu.co
***** Doctor en Ciencias Química, Universidad Nacional de Colombia- Bogotá. Profesor asociado del Departamento de Química, Universidad Nacional de Colombia, Bogotá. Correo electrónico erromerom@unal.edu.co
Recibido:
30/01/2013
Aceptado:
06/09/2013
RESUMEN
In this study, thin films of AgInS2 with chalcopyrite-type tetragonal structure were grown by means of a procedure based on the sequential evaporation of metallic precursors in presence of elemental sulfur in a two-stage process. The effect of the growth temperature and the proportion of the evaporated Ag mass in relation to the evaporated In mass (mAg/mIn) on the phase and homogeneity in the chemical composition were researched through X-ray diffraction measurements and Auger electrons spectroscopy. These measurements evidenced that the conditions for preparing thin films containing only the AgInS2 phase, grown with tetragonal chalcopyrite-type structure and good homogeneity of the chemical composition in the entire volume, are a temperature of 500 °C and a 0.89 mAg/mIn proportion.
The transient photocurrent measurements indicated that the electricity transmission is affected by recombination processes via band-to-band transitions and trap-assisted transitions.
PALABRAS CLAVE
XRD, AES, solar cells, thin films
ABSTRACT
En este trabajo se crecieron películas delgadas de AgInS2 con estructura tetragonal tipo calcopirita usando un procedimiento basado en la evaporación secuencial de precursores metálicos en presencia de azufre elemental, en un proceso en dos etapas. Se investigó el efecto de la temperatura de crecimiento y la proporción de la masa de Ag evaporada a la masa de In evaporada (mAg/mIn) sobre la fase y la homogeneidad en la composición química a través de medidas de difracción de rayos X y espectroscopía de electrones Auger. Estas medidas mostraron que las condiciones para preparar películas delgadas que contengan únicamente la fase AgInS2, crecidas con estructura tipo calcopirita tetragonal y buena homogeneidad de la composición química en todo el volumen son temperatura de 500 °C y proporción mAg/mIn 0.89.
Las medidas de fotocorriente de transiente indicaron que el transporte eléctrico es afectado por procesos de recombinación, vía transiciones banda a banda y transiciones asistidas por trampas.
KEY WORDS
XRD, AES, celdas solares, películas delgadas
INTRODUCTION
AgIn(SxSe1-x)2 based compounds with chalcopyrite type structure have being investigated as potential candidates to be used as absorber layers in single junction and tandem solar cells, due to their high absorption coefficients α (> 104 cm-1) and their band gaps Eg which vary between 1.24 eV (AgInSe2) and 1.96 eV (AgInS2) [1].
Thin films based on the quaternary AgIn(S,Se)2 compound are suitable for absorber layers of single junction solar cells because is possible to grow them with an energy band gap Eg value of 1.45 eV using an adequate S/Se ratio. Semiconductor materials having Eg values close to 1.45 eV are considered optimal for single junction solar cells applications [2]. However, the main advantage of AgIn(S,Se)2 based compounds appears when using them as absorber layers in two junction tandem solar cells, because theoretical calculations indicate efficiencies greater than 30% can be achieved with two junction tandem solar cells fabricated using AgInS2 (1.93 eV) as absorber layer in the top cell and AgInSe2 (1.37 eV) as absorber layer in the bottom cell [2].
AgInS2 (AIS) is the only one amid the I–III-VI2 compounds that could exist in two stable phases: chalcopyrite and orthorhombic. The orthorhombic phase is stable at high temperatures (T> 620°C), and the chalcopyrite phase is stable at low temperatures (T< 620°C) [3]. The AgInS2 films studied in this work were grown in the chalcopyrite phase.
Various techniques have been used to prepare thin films based on Ag-III–VI2 compounds. These include flash evaporation [4], thermal evaporation [5], electro-deposition [6] and solution growing [7]. In this work, p-type AgInS2 thin films with tetragonal chalcopyrite-type structure were grown by co-evaporation of their precursors in a two-stage process. Special emphasis was placed on studying the effects of the growth temperature and mAg/mIn ratio on the chemical composition, crystalline structure and photoconductive properties of AIS thin films.
1. EXPERIMENTAL
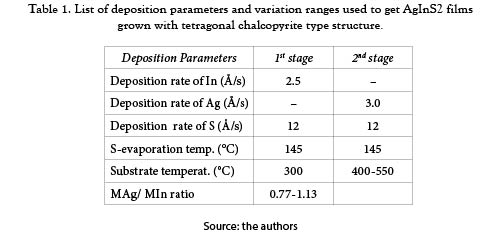
The AgInS2 thin films were deposited on soda lime glass substrates using a procedure based on the sequential evaporation of the metallic precursors in presence of elemental sulphur, in a two stage process. Two separated tungsten boats were used to evaporate Ag and In and a tantalum effusion cell to evaporate sulphur. A thickness monitor (Maxtec TM-400) with a quartz crystal as sensor was used for measuring the deposition rate of the metallic precursor elements and the sulphur flux was controlled by controlling the sulphur evaporation temperature, using a PID temperature controller. The deposition of the AgInS2 films was accomplished in two stages: in the first stage an In2S3 layer was grown by co-evaporation of In and S, keeping the substrate temperature around 300°C. In the second stage, the substrate temperature was elevated to around 500°C and subsequently Ag and S were co-evaporated. Finally, the samples were subjected to a post-deposition thermal annealing in S-ambient during about 60 minutes around 500°C, in order to improve the stability of the chemical composition.
To find conditions for depositing films in the AgInS2 phase with chalcopyrite type structure, several samples were prepared varying the main deposition parameters in a wide range. Initially, the deposition rate of the precursors was varied in a wide range keeping constant both, the substrate temperature during the 1st and 2nd stage (in 300°C and 500°C respectively) and the (mAg/mIn) ratio in 1.00. Using X-ray diffraction (XRD) and transmittance measurements as diagnostic methods, it was found that AgInS2 films with good properties could be obtained by keeping the rate of In and Ag around 2.5 and 3.0 Å/s respectively and the S-evaporation temperature in 145°C (the rate around 12 Å/s). Subsequently, the substrate temperature during the 2nd stage was varied between 400°C and 550°C keeping constant the other parameters as indicated in Table 1.
The elemental evaporated metal flux and the substrate temperature profile that allowed us to grow AgInS2 films with chalcopyrite structure and good optical and structural properties are shown in Figure 1.
The XRD measurements were performed using the Cu Kα radiation of a Shimadzu–6000 difractometer and the Auger Electron Spectroscopy (AES) measurements were carried out using the Perkin-Elmer ESCA/SAM model 560 system. The film thickness was measured with a Veeco Dektak 150 Surface Profiler. Thermoelectric power measurements revealed most of the studied AgInS2 films showed p type conductivity.
The photocurrent measurements were carried out illuminating the samples with a 300 W Halogen lamp, keeping the irradiance intensity at 520 W/m2. The dark current Id and the photocurrent Iph were measured with a picoammeter (Keithley 485) at a constant voltage. The measurements were performed with a data acquisition system developed using virtual instrumentation and the data were processed with a Virtual Instrument (program implemented with LabView) [8] to achieve the Iph vs. t curves during the rise and decay of the photocurrent.
2. RESULTS AND DISCUSSIONS
2.1 Structural characterization
Figure 2 displays typical diffractograms of AgInS2 thin films grown varying the substrate temperature between 400°C and 550°C and the mAg/mIn ratio between 0.77 and 1.13. The experimental diffractograms are compared with a simulated diffractogram obtained using the PowderCell software, which uses a procedure based on the method of Rietveld refinement [9]. From the data reported by ICDD (International Center for Diffraction Data) data base and information obtained from the simulation, we were able to identify the phases present in the samples with a good degree of reliability.
The XRD measurements revealed the AgInS2 films tend to grow with a mixture of the orthorhombic and tetragonal phases; however, we found through a parameter study conditions to prepare AgInS2 thin films containing only the tetragonal phase. Samples prepared using the routine shown in Fig. 1 and a mAg/mIn ratio of 0.89 present reflections associated only to the tetragonal phase (JCPDS #00 025 1330). In samples deposited at temperatures different from 500°C and/or mAg/mIn ratios different from 0.89, reflections associated to the orthorhombic phase were identified. The lattice constants of the AgInS2 films grown with chalcopyrite type tetragonal structure, calculated using the Rietveld refinement method, are: a= 5.8980 Å and c= 11.1935 Å.
2.2 AES depth profile analysis
Figure 3 compares AES depth profiles taken to AgInS2 thin films deposited with two different mAg/mIn ratios and at two different substrate temperatures.
The AES measurements revealed that in general the chemical composition and the homogeneity of the atomic concentration in the bulk of the AgInS2 films are affected by both, the growth temperature and the mAg/mIn ratio. However, we have found that samples prepared at temperatures around 500°C using mAg/mIn ratios near 0.89, grow with chemical composition close to the stoichiometric composition, being their atomic concentration homogeneous in the whole volume (see figure 3a). It was also found that the AIS films prepared at 500°C but using a mAg/mIn ratio of 1.00 present homogeneous chemical composition in most of the bulk; however, in the region close to the substrate the sample becomes Ag-rich and S-poor because in this region may be favoring the formation of sulfide Ag 1+ or 3+ (see fiigure 3b). On the other hand, the AES analysis carried out on samples prepared at temperatures greater than 500°C (keeping mAg/mIn = 0.89), indicated that this type of samples are inhomogeneous in chemical composition (see figure 3c). This behavior could be attributed to the formation of secondary phases like InS2, identified through XRD measurements.
2.3 Photocurrent measurements
Typical AgInS2 films were characterized through photocurrent (Iph) measurements in order to study the nature of the recombination processes. Figure 4 shows the photocurrent rise and decay for a representative AIS film prepared using a mAg/mIn ratio of 0.89, evaporated at a substrate temperature of 500°C, as well as a curve of ln (IPh) vs t.
It is observed that the photocurrent curve deviates from exponential behavior, indicating participation of traps in the carrier generation and recombination processes. It is also observed that the photocurrent curves during illumination and decay are not symmetric, indicating that the resulting recombination mechanism is bimolecular. It is also observed that the photocurrent steady state is reached in a very short time, suggesting a probable presence of β type traps, which are characterized by reaching very rapidly the equilibrium between the occupation of the trapping levels and the conduction band's states and by having small recombination rates. It is also observed that the time constant of the photocurrent decay curve is very small indicating the density of free carriers is larger than the one for trapped carriers; thus the recombination process is dominated by recombination via band to band transitions.
Figure 4b shows a plot of ln (Iph) vs. t curve carried out under an illumination intensity of 520 W/m2. This curve would be a straight line in case of single trap levels. However, in this case the curve presents two different slopes, being the slope in the initial relaxation stage significantly greater than that of the last part of the relaxation curve, indicating the time constant in the initial stage of decay is significantly lower compared with that of the last stage of the relaxation curve. A possible explanation of these results could be given assuming that the first stage of the photocurrent decay is dominated by band to band recombination processes, whereas in the second stage, the photocurrent decay is additionally affected by trap assisted recombination.
Further analysis of the photocurrent measurements was done simulating the Iph(t) vs. t curves with the help of a phenomenological model assuming the linear recombination as the dominant process. In this case the rise and decay curves are explained with the help of multiple component exponentials.
When the above requirements are met, the rise and decay curves are expressed by
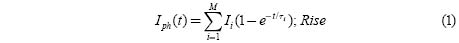
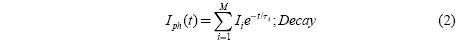
Where Iph is the photocurrent, τi the time constant for a particular process, Ii the contribution of the ith trapping center and M the number of contributing components.
In the present case a good fit to the rise and decay curves was obtained assuming two recombination centers and using a method based on the non-linear least squares regression (NLS) [10]. The basis of the method is to approximate the model by a linear one and to refine the parameters by successive iterations, using as convergence criterion that the relative variation of the sum of the squares from one iteration to the next one is smaller than 0.0001.
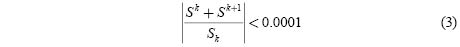
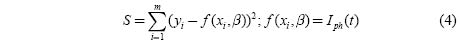
Where Ii and β are the parameters to be refined.
In figure 5 are compared the experimental rise and decay photocurrent curves with the simulated one. The inset displays the values of the parameters (Ii, τi) obtained for each one of the two recombination centers contributing to the whole process. These results reveal the time constants during the rise are different from those calculated during the decay and that the contribution of the 1st recombination center (band to band recombination) to the whole photocurrent is significantly greater than that of the 2nd recombination center (trap assisted recombination). This behavior is more pronounced during the decay than during the rise, indicating the generation rate in both types of recombination centers is quite different from the corresponding recombination rate, probably due to the bimolecular nature of the recombination mechanism. Bimolecular recombination process may occur in highly doped semiconductors which are used as absorbent layer in solar cells.
3. CONCLUSIONS
AgInS2 thin films were grown by sequential evaporation of the metallic precursors in presence of elemental sulphur, in a two stage process. The results revealed the growth temperature and the mAg/mIn ratio significantly affect the phase, crystalline structure and chemical composition of the AIS films; however, through an exhaustive parameter study, conditions were found to grow AgInS2 films of homogeneous chemical composition with chalcopyrite type tetragonal structure. AES measurements revealed the AIS films prepared at temperatures around 500°C and using mAg/mIn ratios near 0.89 grow with chemical composition close to the stoichiometric composition, and their atomic concentration is homogeneous in the whole volume.
Measurements and simulation of the transient photocurrent indicated that the electrical transport in AIS films is affected by recombination processes via band to band transitions and trap assisted transitions, being the band to band recombination the dominant one.
Acknowledgements: The authors would like to thank Universidad Nacional de Colombia and Eng. Wilian Cauich for his assistance with the AES measurements.
REFERENCES
[1] K. Yoshino, N. Mitania, M. Sugiyamab, S. F. Chichibub, H. Komakia and T. Ikari, Optical and electrical properties of AgIn(S,Se)2 crystals'', Physica B, vol. 302-303 pp. 349-356, 2001.
[2] S. M. Patel and A. D. Patel, Growth of AgInSe2 thin films'', Thin Solid Films, vol. 111, no. 1, pp. 53-58, 1984.
[3] J. L. Shay, B. Tell, L.M. Schiavone, H. M. Kasper and F. Thiel, Energy bands of AgInS2 in the chalcopyrite and orthorhombic structures'', Phys. Rev. B. vol. 9, no. 4, pp. 1719-1723, 1974.
[4] R. D. Weir, P.E. Jessop and B.K. Garside, Growth and annealing of AgInSe2 thin films'', Canadian Journal of Physics, vol. 65, no. 8, pp. 1033-1036, 1987.
[5] Y. Ueno, Y. Kojima, T. Sugiura and H. Minoura, Electrodeposition of AgInSe2 films from a sulphate bath'', Thin Solid Films, vol. 189 No.1, pp. 91-101, 1990.
[6] R. P. Sharma, Influence of annealing in vacuum on opto-electronic characteristics of solution grown AgInSe2 films'', Indian J. Pure & Appl. Phys, vol. 33, no. 11, pp. 711-713, 1995.
[7] J. L. Shay and J.H. Wernick, Ternary Chalcopyrite Semiconductors: Growth, Electronic Properties and Applications, Oxford, New York, Pergamon Press, 1975, 244 p.
[8] G. W. Johnson and R. Jennings, LabVIEW, Graphical Programming. 3rd ed, Austin, McGraw-Hill, 2006, 752 p.
[9] H. M. Rietveld, A profile refinement method for nuclear and magnetic structure'', J. Appl. Crys, vol. 2 pp.65-71, 1969.
[10] J. Fox, An R and S-plus companion to applied regression, Thousand Oaks, California, Sage Publications, 2002, 328 p.